10 Common Chemical Reactions That Occur in Everyday Life
Look at a few chemical reactions that are common in daily life, but you might not have noticed them until now. Do you know that chemical reactions occur when you bake or cook?

What comes in your mind when you hear the word chemistry? I imagine myself standing in a chemistry lab doing some experiments. If you are a science student, then you would agree that spending time in labs is better than sitting on a chair and reading theory. Trying and testing certain things with chemicals and telescope is fun. But do you know chemical reaction does not only happen in chemistry labs, but several chemical reactions happen every second outside the lab also?
A certain reaction happens even when you sleep or wake up in the morning. You won’t believe it, but all your daily activities, including cooking, drinking water, and others, are carried by different chemical processes. If you and your kid love experimenting, then these easy science experiments can be tried with kids at home. Let’s now see some chemical reactions that you experience every day but might not notice.
1. Photosynthesis
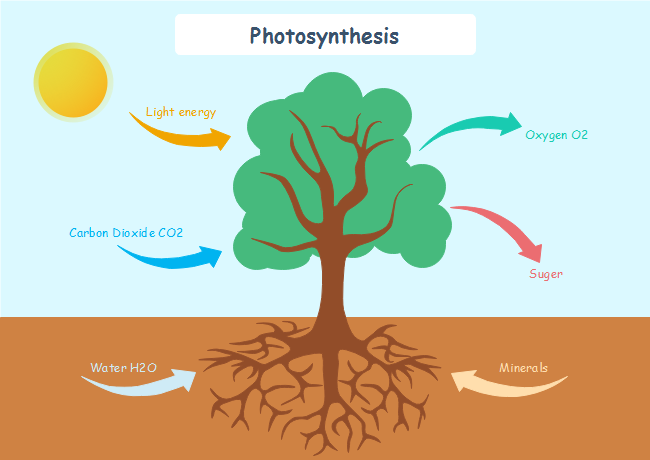
Plants convert carbon dioxide and water into glucose and oxygen through the process of photosynthesis. In the presence of chlorophyll and sunlight, plants take carbon dioxide and water from the environment.
It is one of the best examples of chemical reactions that can be noticed in everyday life. This is how plants produce food for themselves. During this process, light energy is captured and is used to convert minerals into energy-rich organic compounds. The chemical reaction that happens during photosynthesis is:
6CO2 + 6H2O + Sunlight → C6H12O6 + 6O2
2. Rusting

You must have seen brownish flaky coating over the metal surfaces. This brown coating on iron is formed due to the oxidation of the topmost layer. The atoms present in the metal undergo reduction and oxidation reaction and cause rusting.
Tarnishing of silver and verdigris on silver are other common examples of rusting. It is caused by the oxidization of iron in the steel. When the oxygen combines with the metal, it forms a compound that is known as rust. The chemical reaction that happens during this process is:
Fe+3O2+xH2O————->Fe3O4.xH2O
3. Chemical Reactions in Food Preservatives

Have you ever wondered why foods like pickles do not get stale even after keeping them unused for a year? Foods like sauces, pickles, and several beverages contain preservatives so that they remain fresh for a longer time. When you buy these foods, you must have noticed a long-list of chemicals.
They are preservatives and delays the growth of microorganisms in foods. Furthermore, the preservatives also hinder the oxidation, which is responsible for staling the food. The most common preservatives used are calcium sorbate, sodium benzoate, propionic acid, and sucrose.
4. Body Composition
You might not know that carbon and oxygen are the most crucial elements of the body. The other elements that are present in the body are potassium, magnesium, calcium, and nitrogen. The function of oxygen is to regulate temperature and osmotic pressure.
Phosphorus provides enough strength and structure to teeth and bones, Magnesium builds bones and muscles, Aluminum increases the effects of vaccines and medicines, Vanadium metabolizes enzymes, and fluorine is responsible for the formation of tooth enamel.
5. Chopping an Onion is Also a Chemical Reaction

Have you ever wondered why tears come when you chop an onion? This happens due to sulphonic acid that is formed from amino acid sulfoxides. This acid is responsible for volatile gas that produces the tears in the eyes. It contains amino acid sulfoxides that produce sulfenic acids in the cells.
When you cut an onion, the enzymes get mixed with sulfenic acid and produce Propanethial S-oxide. It is a chemical that floats through the air and then into your eyes. Also, cooked onions don’t produce tears because when onion gets cooked, the enzymes get inactive that are needed to make Propanethial S-oxide.
6. When You Apply the Sunscreen

It is recommended to apply sunscreen whenever you go out during sunny hours. Have you ever wondered why? You might be surprised after reading this that the working of sunscreen is also associated with chemistry. Any sunscreen lotion that you buy from the market contains several organic and inorganic compounds that act as a filter for the ultraviolet rays.
Inorganic chemicals, like zinc oxide or titanium oxide, act as a sunblock. These chemicals scatter the UV rays away from the skin and protect it from sun rays. Sunscreen not only prevents the skin from UV rays, but it also reduces the risk of skin cancer.
7. Cooking and Chemical Reaction

Chemical reactions in cooking make the food tastier. Have you ever thought why freshly baked bread smells so good? Or how green berries turn into aromatic brown coffee beans? These processes also have a series of chemical reactions that are known as the Maillard reaction.
This reaction produces chemical compounds that give aroma and color to foods like potatoes, meat, chocolate, and other products. The reaction occurs between amino acids and occurs mostly when there is low moisture or when the temperature is above 130℃. The color of fudges and chocolates are produced due to the reaction of sugars and milk protein.
Caramelization, which occurs at 356 degrees F, is the last reaction that happens during the baking process. It occurs when heat causes molecules to break down and then to turn into steam. After a certain stage, the production of molecules gives a nutty flavor and produces a toasty flavor.
8. When You Wash Your Hands With Soap

You wash your hands with soap before you cook or consume food. The soap contains water, oil, and basic salt. Also, soap is the fatty acid salt of potassium and is produced by a chemical reaction known as saponification. When soap interacts with oil molecules, it results in a cleaner surface.
Today, the soaps are produced through two processes - cold and hot. In the hot process, the ingredients are heated when they are mixed and increase the speed of the saponification process. In the cold process, the Iye solution is mixed with vegetable oil. As the ingredients are mixed, the mixture thickens and is poured into the mold.
Some think that antibacterial soap is better than regular soap. Antibacterial soap has ingredients like triclocarban that penetrates the bacterial cell membrane and kills the bacteria. But studies showed that antibacterial soaps are not effective in comparison to regular soap.
9. Chemistry of Emotions
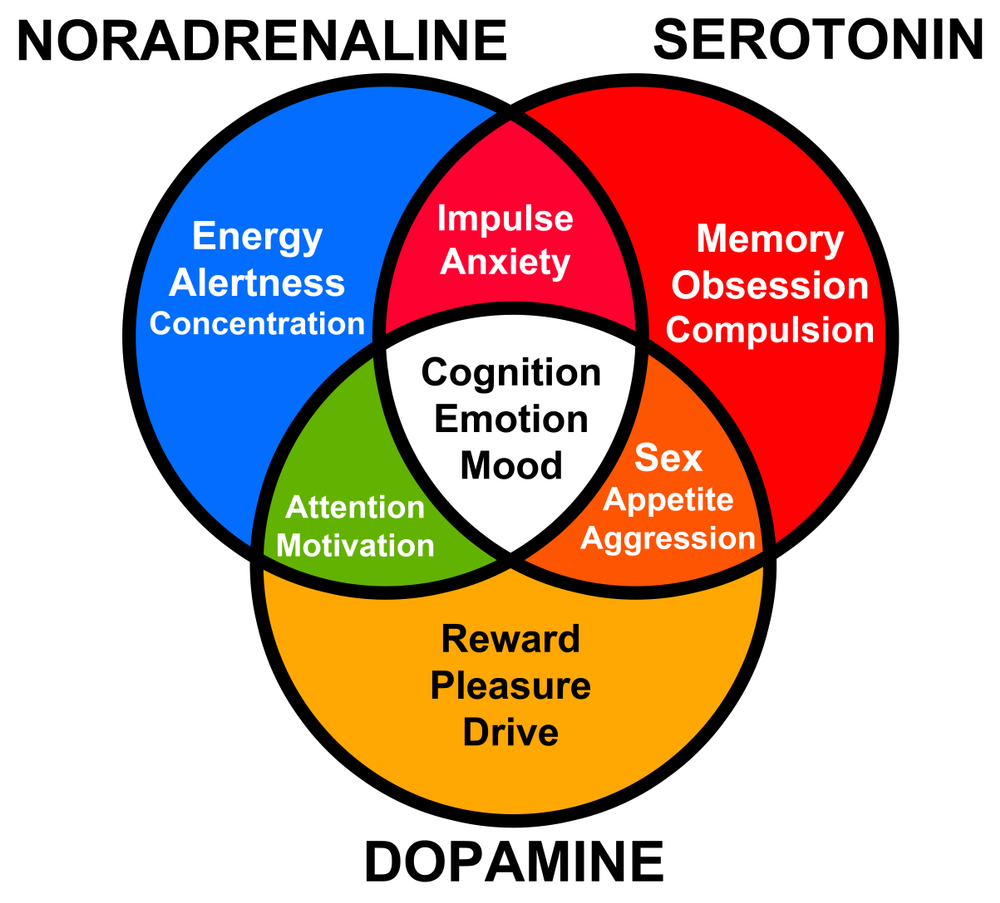
Do you know when you are happy, sad, or excited, several chemical reactions take place in your body? These reactions occur in the body because of the chemical messengers that are released in the brain. Endorphins also released when you play video games. It is released when you are overcoming challenges and accomplishing new goals.
Oxytocin creates social bonds and causes a feeling of connection. It is released when you make eye contact with someone you love. Dopamine is a neurotransmitter and is released by the hypothalamus. It is involved in muscle control, memory drive, and ovulation.
GABA (gamma-aminobutyric acid) is a neurotransmitter that is produced from an amino acid and slows down the activity of the limbic system, anxiety, and pain.
You experience a range of emotions throughout the day. Sometimes these emotions negatively affect your body; other times, they share positivity. Now, do you understand why you need someone when you are in a bad mood or why you easily deal with challenges when you are happy?
10. Metathesis

If you mix vinegar and baking soda, there happens a double displacement reaction or metathesis reaction. The ingredients combined to produce CO2 and water. The CO2 forms bubble and helps in the process of baking. This reaction seems simple but consists of multiple steps.
The chemical reaction that takes place is:
HC2H3O2(aq) + NaHCO3(aq) → NaC2H3O2(aq) + H2O(l) + CO2(g)
Final Words
You don’t have to go to the chemistry lab to try these experiments. Instead, all the above chemical reactions can take place anywhere in everyday life.
Next time, when you cut an onion or wash your hands, recall the reactions that you have read in the chemistry books. Here are some science experiments that you could try at home to better know how science works.
What else have you experimented in chemistry? Have you tried or observed any of these chemical reactions? If so, then don’t forget to share your reactions.
Popular Posts
What Is Trypophobia – A Disgust More Than Fear
"I can't really face small, irregularly or asymmetrically placed holes, they make me like, throw up in my mouth, cry a little bi...
Chandan Roy
16 Interesting Facts About Ambidextrous People
A lefty or left-handed uses his left hand more naturally and dominantly than the right hand. And the righty or right-handed is o...
Ethan Stephans
20 Interesting Facts About Meteoroid, Meteor and Meteorite
Watching celestial objects is a true delight. It is still fun to catch a sight of shooting stars when we grow up. A second of th...
Swati Bhandari








